LiMan Collaboration|LiMan ubiquitination enrichment antibody products support the clinical application potential of SCASP-PTM technology in 《Cell Reports》
2025-04-18
Background
In modern life science research, post-translational modifications (PTMs) are increasingly recognized as playing a central role in regulating cellular function and signal transduction. PTMs alter the activity, stability, and interactions of proteins through chemical modifications, thereby affecting fundamental biological processes such as cell growth, differentiation, and apoptosis. These modifications include various forms such as phosphorylation, acetylation, ubiquitination, and glycosylation, and they play an indispensable role in cell biology and cancer research.
Leman ELEMAb series antibodies The ubiquitination enrichment antibody (Catalog Number: LMMSPTM0300) was used in this study. For details, please see : Product Launch | Ubiquitination Enrichment Antibody Series in the field of protein degradation drugs Welcome to contact us for a trial kit.
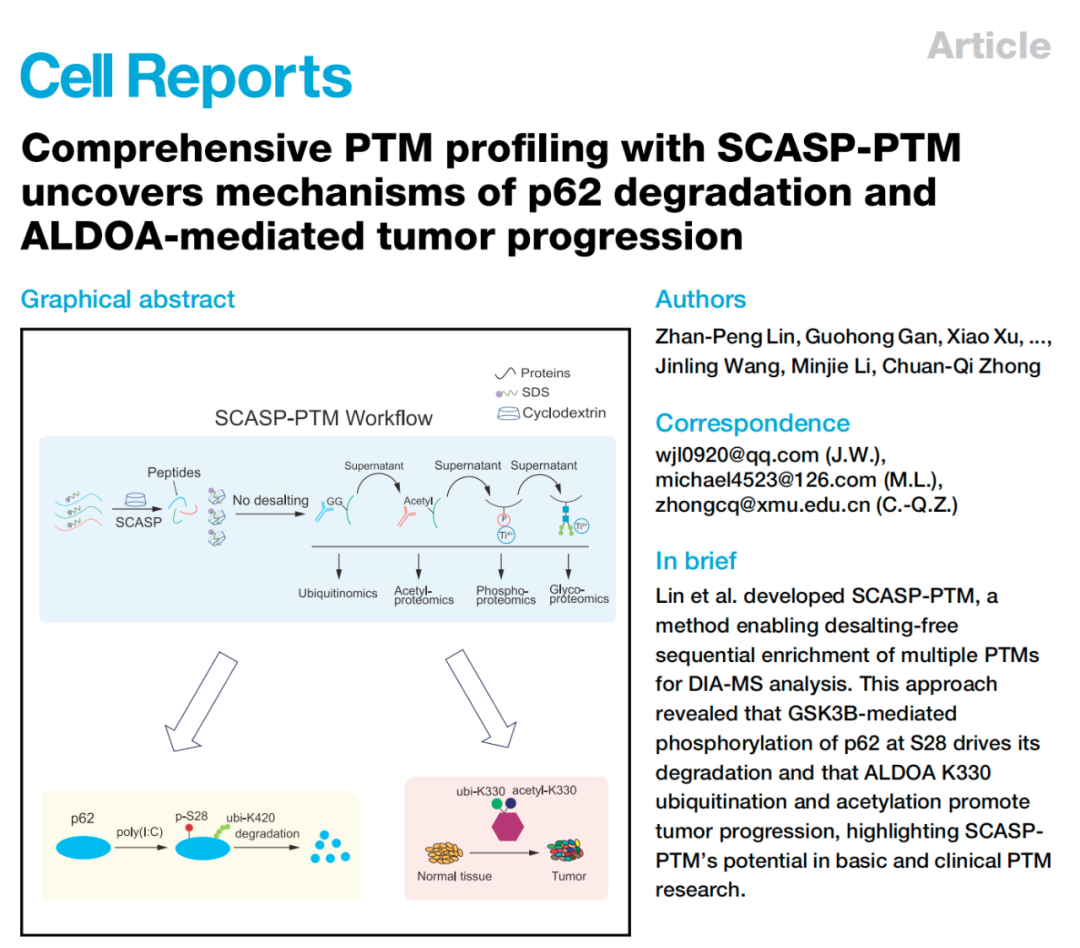
1. SCASP-PTM: An innovative technology for multi-PTM analysis
A recent article published in Cell Reports details a new technology called SCASP-PTM (SDS Cyclodextrin Assisted Sample Preparation for PTM). This technology enables salt-free, continuous enrichment of multiple PTMs in samples without complex purification steps, facilitating data-independent acquisition mass spectrometry (DIA-MS) analysis. The core of SCASP-PTM technology lies in its highly efficient sample preparation process, utilizing cyclodextrin to bind SDS and eliminate the effects of protein denaturants, thereby achieving efficient enrichment of multiple PTMs.
Traditional PTM analysis methods typically require separate sample processing and analysis for each modification, which is not only time-consuming but also prone to sample loss and analytical errors. SCASP-PTM technology integrates multiple enrichment methods, such as TiO2, IMAC, and CaTiO3, to enable simultaneous analysis of multiple modifications such as phosphorylation, acetylation, ubiquitination, and glycosylation. This innovative method significantly improves experimental efficiency and data accuracy, providing a powerful tool for PTM research.
2. New discoveries in the p62 protein degradation mechanism
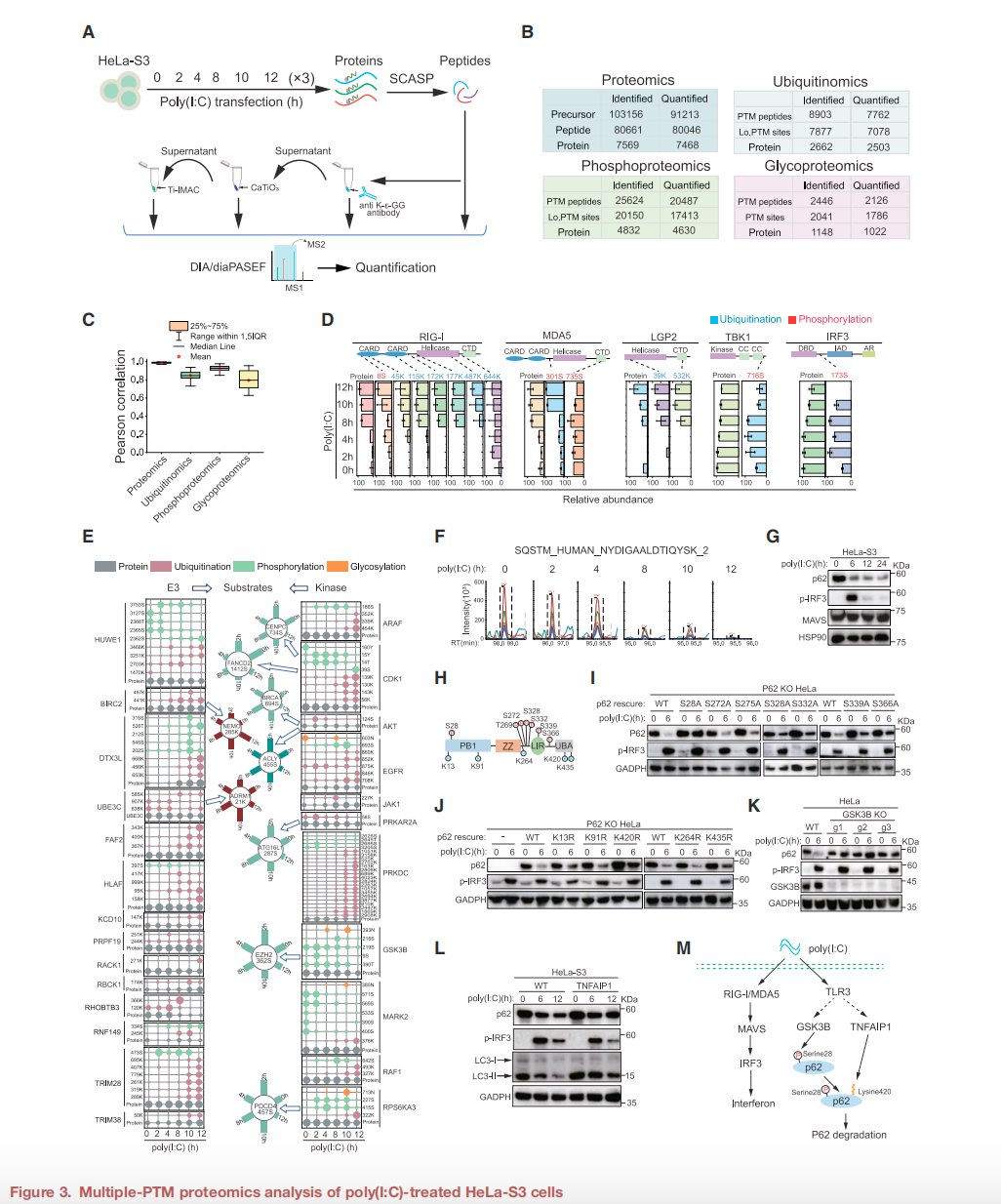
In cells, p62/SQSTM1 protein is an important autophagy receptor that plays a key role in cellular homeostasis by mediating protein degradation and signal pathway regulation. Studies have shown that abnormal expression of p62 is closely related to various diseases, including neurodegenerative diseases and cancer. In this study, SCASP-PTM technology revealed that GSK3B-mediated phosphorylation of p62 at position S28 is a driving factor for its degradation. Through experiments on HeLa-S3 cells, researchers found that phosphorylated p62 is more easily degraded by the proteasome, and the proteasome inhibitor MG132 can block this process.
This finding provides a new perspective on understanding the complex mechanisms of protein degradation and may provide a reference for developing related therapeutic strategies. The degradation of p62 involves not only its own phosphorylation status but may also be related to the interaction with other signaling pathways. Future research can further explore the specific mechanisms of these interactions to gain a more comprehensive understanding of the role of p62 in cellular physiological and pathological states.
3. The relationship between ALDOA protein and tumor progression
In a multi-PTM proteomics study of lung cancer, the K330 site of ALDOA (aldolase A) protein was found to have significant ubiquitination and acetylation modifications. ALDOA is a glycolytic enzyme that plays an important role in the metabolic reprogramming of cancer cells. Studies have shown that these modifications of ALDOA are key drivers of tumor growth and progression.
By analyzing lung cancer tissue samples, researchers found that the expression level of ALDOA in tumors was significantly higher than that in normal tissues, and the modification level of its K330 site was also significantly upregulated. Further functional experiments showed that the K330R mutation can enhance the enzymatic activity of ALDOA and slow down cell growth and migration. This finding reveals the important role of ALDOA in tumor cell proliferation and migration and provides a theoretical basis for its potential as a therapeutic target.
Ubiquitination and acetylation modifications of ALDOA may promote the metabolic adaptation and invasion ability of tumor cells by regulating its enzymatic activity and protein interaction network. Future research can further explore the role of ALDOA in different types of tumors and how its modification status affects the biological behavior of tumors.
4. Potential for clinical application
The clinical application potential of SCASP-PTM technology is mainly reflected in the following aspects:
- Discovery of biomarkers: Through systematic analysis of PTMs in tumor tissues and cell samples, SCASP-PTM technology can help identify new biomarkers. These biomarkers can not only be used for early diagnosis and prognosis assessment but may also provide guidance for personalized treatment.
- Identification of therapeutic targets: PTMs play an important role in regulating protein function and signaling pathways. By revealing the molecular mechanisms of signaling pathways, SCASP-PTM technology may help identify new therapeutic targets. This is of great significance for the development of drugs targeting specific PTMs.
- Study of drug mechanisms of action: By analyzing changes in PTMs in cells or tissues before and after drug treatment, SCASP-PTM technology can be used to study the mechanisms of action of drugs. This will help optimize existing treatment regimens and develop new drugs.
- Advancement of personalized medicine: SCASP-PTM technology can perform detailed analysis of PTMs in patient samples, thereby providing data support for personalized treatment. By identifying patient-specific PTM patterns, doctors can choose the most appropriate treatment regimen to improve treatment outcomes.
5. Challenges and future directions
Although SCASP-PTM technology has shown great potential in PTM research, it still faces some challenges. First, the technology mainly targets common PTM types, while its enrichment efficiency and detection sensitivity for some rare or low-abundance PTMs still need to be further improved. Second, the dynamic and complex nature of PTMs requires consideration of multiple factors in experimental design and data analysis to ensure the accuracy and reproducibility of the results.
Future research can further enhance the application value of SCASP-PTM technology through the following directions:
- Technical optimization: Improve the detection ability of low-abundance and rare PTMs by improving sample preparation and enrichment methods. Combine new mass spectrometry technologies to improve the sensitivity and resolution of the analysis.
- Development of data analysis tools: With the accumulation of PTM data, develop more advanced data analysis tools and algorithms to better process and interpret complex PTM data. This will help reveal the functions and mechanisms of PTMs in biological processes.
- Multi-omics integrative analysis: Combining SCASP-PTM technology with multi-omics data such as genomics, transcriptomics, and metabolomics for comprehensive analysis. This will help build a more comprehensive biological network and reveal the global role of PTM in cell function regulation.
- Clinical translational research: Closely integrating with clinical research to evaluate the practical application effects of SCASP-PTM technology in disease diagnosis, treatment, and prognosis. Promoting the translation of this technology from laboratory research to clinical application.
Summary and Outlook
SCASP-PTM technology provides a new perspective for understanding tumor biology and demonstrates its broad application potential in post-translational modification research. With continuous improvement and expansion of its application scope, SCASP-PTM is expected to play a greater role in clinical diagnosis and treatment, contributing to overcoming the medical challenge of tumors. In the future, researchers will continue to explore the application potential of SCASP-PTM technology in other diseases and strive to develop more efficient and precise PTM analysis methods. Through close integration with clinical research, SCASP-PTM technology is expected to open up new avenues for personalized medicine and precision treatment.
References
Lin ZP, et. Comprehensive PTM profiling with SCASP-PTM uncovers mechanisms of p62 degradation and ALDOA-mediated tumor progression. Cell Rep. 2025 Apr 3; 44(4):115500. doi: 10.1016/j.celrep.2025.115500. Epub ahead of print. PMID: 40186868.
2025 /
04-18
Classification:
Company News
Related Information


